Investigation, development and correlation of novel differentiation and inflammation models based on in-vitro-co-culture and in-vivo-investigation in the context of biocompatibility demonstration and extension of a valid ex-vivo-test bench
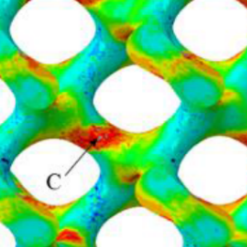
The main goal of this sub-project is the acquisition, validation and analysis of relevant clinical data regarding the Ti-6Al-4V alloy in vitro, in vivo and ex vivo, as a basis for the optimization of the testing material for clinical use. The aim is to determine cytocompatibility in vitro using human dental pulp stem-cells (hdPC) in specialized cyto- and biocompatibility analyses, to fulfill specific requirements in oral and maxillo-facial surgery. Further analysis to characterize material properties with regards to inflammatory processes shall also be performed by the use of multicolor flow-cytometry. In addition to the invitro results, a subsequent in vivo biocompatibility analysis will be carried out on the basis of an animal model (Goettinger miniature pigs). The purpose of the in vivo assay will be to evaluate micromorphological effects of additively manufactured implant materials on the surrounding tissue as well as to measure the influence of different defect models and various durability. Additionally, the effect of tissue integration shall be characterized generally as well as stage-dependently with regards to long-term implant stability and to the implant-tissue-interface. Further mechanical examinations will also be carried out on suitable explanted implant-bone-combinations. In view of the specific requirements in the field of oral and maxillo-facial surgery (field of application of dental implants, osteosynthesis material (plates, screws)) and patient-specific implants (scull cap, orbital floor), the biomechanical load will be represented in the test bench in close cooperation with SP-4 according to DIN EN ISO 14801 under physiological conditions, i.e. in an artificial saliva medium at 37 °C, to compare the aging process of the test specimens with data from animal testing.